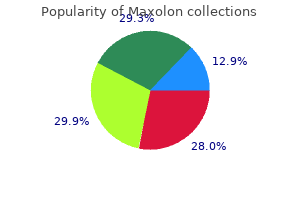
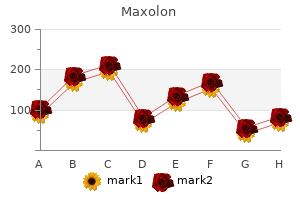
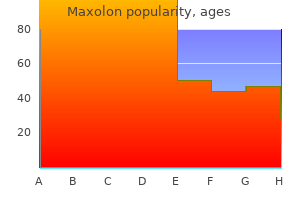
Maxolon
Edgar D. Staren, MD, PhD, MBA
- Senior Vice President and Chief Medical Officer
- Cancer Treatment Centers of America
- Zion, Illinois
- Visiting Professor
- Department of General Surgery
- Rush Medical College
- Chicago, Illinois
Parenteral Iron Therapy Intravenous iron can be given to patients who are unable to tolerate oral iron; whose needs are relatively acute; or who need iron on an ongoing basis gastritis diet ���� , usually due to persistent gastrointestinal blood loss gastritis from coffee . Fortunately gastritis diet ���� , newer iron complexes are available in the United States gastritis symptoms bloating , such as sodium ferric gluconate (Ferrlecit) and iron sucrose (Venofer), that have a much lower rate of adverse effects. Parenteral iron is used in two ways: one is to administer the total dose of iron required to correct the hemoglobin deficit and provide the patient with at least 500 mg of iron stores; the second is to give repeated small doses of parenteral iron over a protracted period. The amount of iron needed by an individual patient is calculated by the following formula: Body weight (kg) Ч 2. The factors that have correlated with an anaphylactic-like reaction include a history of multiple allergies or a prior allergic reaction to dextran (in the case of iron dextran). Generalized symptoms appearing several days after the infusion of a large dose of iron can include arthralgias, skin rash, and low-grade fever. This may be dose-related, but it does not preclude the further use of parenteral iron in the patient. To date, patients with sensitivity to iron dextran have been safely treated with iron gluconate. If a large dose of iron dextran is to be given (>100 mg), the iron preparation should be diluted in 5% dextrose in water or 0. The iron solution can then be infused over a 60- to 90-minute period (for larger doses) or at a rate convenient for the attending nurse or physician. While a test dose (25 mg) of parenteral iron dextran is recommended, in reality a slow infusion of a larger dose of parenteral iron solution will afford the same kind of early warning as a separately injected test dose. Early in the infusion of iron, if chest pain, wheezing, a fall in blood pressure, or other systemic symptoms occur, the infusion of iron should be stopped immediately. For the anemia of chronic inflammation, the erythroid marrow also responds inadequately to stimulation, due in part to defects in iron reutilization. This is reflected by a low serum iron, increased red cell protoporphyrin, a hypoproliferative marrow, transferrin saturation in the range of 1520%, and a normal or increased serum ferritin. The serum ferritin values are often the most distinguishing feature between true iron-deficiency anemia and the iron-deficient erythropoiesis associated with inflammation. Typically, serum ferritin values increase threefold over basal levels in the face of inflammation. All of these changes are due to the effects of inflammatory cytokines and hepcidin, the key iron regulatory hormone, acting at several levels of erythropoiesis. Hepcidin, made by the liver, is increased in inflammation and acts to suppress iron absorption and iron release from storage sites. The overall result is a chronic hypoproliferative anemia with classic changes in iron metabolism. The anemia is further compounded by a mild to moderate shortening in red cell survival. With chronic inflammation, the primary disease determines the severity and characteristics of the anemia. For instance, many patients with cancer also have anemia that is typically normocytic and normochromic. The fever and cytokines released exert a selective pressure against cells with more limited capacity to maintain the red cell membrane. In most individuals the mild anemia is reasonably well tolerated, and symptoms, if present, are associated with the underlying disease. Occasionally, in patients with preexisting cardiac disease, moderate anemia (hemoglobin 10-11 g/dL) may be associated with angina, exercise intolerance, and shortness of breath. Table 7-6 shows the erythropoietic profile that distinguishes the anemia of inflammation from the other causes of hypoproliferative anemias. Red cells are typically normocytic and normochromic, and reticulocytes are decreased. In certain forms of acute renal failure, the correlation between the anemia and renal function is weaker. Patients with the hemolytic-uremic syndrome increase erythropoiesis in response to the hemolysis, despite renal failure requiring dialysis. Assessment of iron status provides information to distinguish the anemia of renal disease from the other forms of hypoproliferative anemia (Table 7-6) and to guide management.
Quinine-induced immune thrombocytopenia associated with hemolytic uremic syndrome gastritis upper right back pain . Quinineinduced immune thrombocytopenia with hemolytic uremic syndrome: clinical and serological findings in nine patients and review of literature gastritis diet ��������� . Sunitinib induced hypertension gastritis anxiety , thrombotic microangiopathy and reversible posterior leukencephalopathy syndrome gastritis diet ���� . Thrombotic microangiopathy in the cancer patiлnt including those induced by chemotherapeutic agents. Transplantation-associated thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Incidence and clinical course of thrombotic thrombocytopenic purpura due to ticlopidine following coronary stenting. Interventions for haemolytic uraemic syndrome and thrombotic thrombocytopenic purpura. Set up Following a general introduction about adverse effects of transfusion of blood components and the differential diagnosis and treatment of acute transfusion reactions (7. Chapter 9 discusses the requirements that a hospital must meet concerning the monitoring of the transfusion chain, such as having access to a functioning blood transfusion committee, a haemovigilance official, a haemovigilance employee, and a training and further education program for all those involved in the transfusion chain. For this guideline, it was decided to divide according to cause, namely into non-infectious and infectious complications. In addition, the categorisation into acute symptoms during or within 24 hours of the transfusion and non-acute symptoms more than 24 hours after the transfusion is also used. All acute reactions except those due to bacterial contamination are non-infectious. Acute reactions require an acute diagnostic and if necessary treatment policy. For this reason, we will first focus on the diagnosis and treatment of acute transfusion reactions. Blood Transfusion Guideline, 2011 279 - component; in the case of sepsis, treat as such and start antibiotics; consider haemolytic transfusion reaction (see paragraph 7. Itching/urticaria: If there are no anaphylactic symptoms (such as glottis oedema, hypotension, shock): consider administering an anti-histamine. The recommendations provided below are based on the opinions of experts and international guidelines (evidence level 4). A nurse must observe the patient for 5 to 10 minutes after starting the transfusion of each new unit. Clearly define which parameters should be monitored (heart rate, temperature, blood pressure, etc. In the case of a (suspected) transfusion reaction other than itching or urticaria, the transfusion should be stopped and the unit disconnected if necessary, in consultation with the treating physician. Rapid and targeted examination by the blood transfusion laboratory is also required. The treating physician should be contacted for the differential diagnosis and treatment of acute transfusion reactions. It is recommended that the treating physician follows the above-mentioned algorithm (7. For more detailed recommendations for (suspected) specific reactions: see paragraph 7. If anaphylactic symptoms (such as glottis oedema, hypotension, shock) are present: disconnect the unit immediately, connect a neutral infusion solution (e. If the blood component is disconnected, it should be returned to the blood transfusion laboratory as soon as possible for further examination. The hospital must provide instructions for disconnection, transport & storage conditions, and the method of sampling and these instructions must be followed. Reporting: Transfusion reactions must first be reported to the treating doctor and the blood transfusion laboratory. Sanquin Blood Supply should be contacted as soon as possible with each suspected transfusion reaction or incident that may have 280 Blood Transfusion Guideline, 2011 8.
Rule gastritis meaning , S: Consultant Advisory Role: Celgene gastritis young living , Roche chronic gastritis histology , Astra Zeneca gastritis length , Janssen, Sunesis; Honoraria: Celgene, Roche, Astra Zeneca, Janssen, Sunesis; Research Funding: Janssen. Johnston, A: Consultant Advisory Role: Yes; Other Remuneration: Support to attend meeting. This trial is currently recruiting, and plans to enrol 875 patients in 24 countries. Flowers, C: Consultant Advisory Role: AbbVie, AstraZeneca, Bayer, BeiGene, Celgene (unpaid), Denovo Biopharma, Genentech, Inc. Friedberg, J: Consultant Advisory Role: Bayer, Astellas Pharma; Research Funding: Seattle Genetics, Kite Pharma; Other Remuneration: Roche (travel, accommodation and expenses); patent on bone marrow microenvironment signals. Herbaux, C: Honoraria: Roche, Janssen-Cilag, AbbVie; Research Funding: Takeda; Other Remuneration: JanssenCilag, AbbVie, Roche (travel, accommodation and expenses). Trnnэ, M: Consultant Advisory Role: Takeda, Bristol-Myers Squibb, Incyte, AbbVie, Amgen, Roche, Gilead Sciences, Janssen, Celgene, MorphoSys; Honoraria: Janssen, Gilead Sciences, Takeda, Bristol-Myers Squibb, Amgen, AbbVie, Roche, MorphoSys, Incyte; Other Remuneration: Gilead Sciences, Takeda, Bristol-Myers Squibb, Roche, Janssen, AbbVie (travel, accommodation and expenses). Subjects randomized to the investigational arm who have a complete or partial response will have the option to continue in the single agent phase to receive enzastaurin at 500 mg/day for up to 2 additional years. Luo, W: Employment Leadership Position: Denovo Biopharma; Stock Ownership: Denovo Biopharma; Research Funding: Denovo Biopharma. Shazer, R: Employment Leadership Position: Inspyr Therapeutics; Denovo Biopharma; Stock Ownership: Bristol-Myers Squibb; Pfizer. Zhang, L: Employment Leadership Position: Celgene; Denovo Biopharma; Stock Ownership: Celgene; Denovo Biopharma. Nowakowski, G: Consultant Advisory Role: Celgene; MorphoSys; Genentech; Research Funding: Celgene; NanoString Technologies; MorphhoSys. LaPlant1 Division of Hematology, Mayo Clinic, Rochester, United States; 2Division of Hematology, Mayo Clinic, Scottsdale, United States; 3Division of Hematology, Mayo Clinic, Jacksonville, United States; 4Siteman Cancer Center, Washington University School of Medicine in St. Phase 1 data supports the safety and tolerability of single-agent varlilumab in advanced hematologic malignancies. Standard inclusion criteria and prior treatment with at least 2 lines of standard therapy are required. Eligible patients will be randomized to treatment with single-agent nivolumab (group 1) or dual immunotherapy with nivolumab and varlilumab. Subsequently a continuation phase of A only, for 2 cycles of 28 days will be administered. The effect of acalabrutinib on antibody-directed cellular cytotoxicity mediated by rituximab will be measured in vitro during treatment. This is an investigator initiated study that has been granted free access to investigational medicinal product, trial management and translational study support through a grant from Acerta Pharma B. Griffiths, G: Research Funding: Hold educational trial grants from numerous companies including AcertaPharma. Johnson, P: Honoraria: Bristol-Myers Squibb, Takeda, Novartis, Celgene, Janssen, Epizyme, Boeringher Ingelheim, Kite, Genmab, Incyte; Research Funding: Janssen, Epizyme. Secondary endpoints include safety, other efficacy endpoints, and biomarker analyses. Disclosures: Porcu, P: Consultant Advisory Role: Innate Pharma; Research Funding: Kyowa Kirin, Viracta. Kim, Y: Honoraria: kyowa Kirin, Eisai, Millennium/Takeda, Seattly Genetics, miRagen, Innate Pharma; Research Funding: Kyowa Kirin, Merck, Soligenix, FortySeven, Neumedicines, Portola Pharma, and Horizon. Sicard, H: Employment Leadership Position: Innate Pharma; Stock Ownership: Innate Pharma. Azim Jr, H: Employment Leadership Position: Innate Pharma; Stock Ownership: Innate Pharma. Bagot, M: Consultant Advisory Role: Innate Pharma; Other Remuneration: Travel fees: Innate Pharma, Kyowa Kirin. Y195H allele and model the loss of Tnfaip3 by using a previously published floxed allele. Y195H expression and loss ofTnfaip3 and initial comparative results will be shown. Morin1 Institute of Pathology, University Hospital of Cologne, Cologne, Germany M.
Syndromes
- Lower in foods that have a lot of sugar, such as soft drinks, fruit juices, and pastries
- Low back pain
- Problems sticking to a regular sleep schedule (sleep rhythm problem)
- Headaches (uncommon)
- Weakness
- Stretch out narrow segments (bile duct strictures)
- General discomfort or uneasiness (malaise)
- Collapse
- High protein diet
Cells specifically regulate the transport of sodium (Na+) gastritis diet journal template , potassium (K+) and calcium (Ca2+) ions across the plasma membrane gastritis treatment , store additional Ca2+ ions within the endoplasmic reticulum gastritis diet ��������� , and effectively sequester K+ within the cytoplasmic compartment symptoms of gastritis and duodenitis . Reactive oxygen species are a natural byproduct of energy production, but are effectively countered by cellular antioxidant mechanisms of defense [8]. From a clinical efficacy standpoint, these environmental stresses are compounded at each step of the cell product lifecycle, introducing sample variability and the potential for impaired therapeutic function, which may even lead to clinical inefficacy and termination of a potentially effective treatment. From a financial standpoint, loss of cell yield, viability and function adds significant cost to cell therapies by increasing the potential for repeat sampling or additional processing steps to expand cell numbers or resuscitate function [4]. Biopreservation refers to the processes required to maintain the health and function of biologics outside the body, as well as suppress the degradation of these biological materials to ensure a return to function post-preservation [5]. In contrast to normothermia, each 10°C decrease in temperature reduces metabolism approximately 50% for energetically active tissue such as the brain [9], minimizing the environmental requirements of cells outside culture conditions. As a result, low-temperature biopreservation, both at 28°C hypothermic temperatures, or cryogenically frozen between -80°C and -196°C, is the most common and preferred method of storage employed in cell therapy and regenerative medicine applications. Despite the metabolic benefits of low-temperature biopreservation, temperature reduction exerts unique stresses that must be carefully addressed to maximize cell viability and function upon a return to normothermia. Cells at reduced temperatures (hypothermic storage and cryopreservation) undergo reduced membrane ion pump activity and a physical reorganization of the plasma membrane that increases permeability [10]. Consequentially, Na+ ions flow into the cell and K+ ions escape into the extracellular compartment [11]. The Ca2+ concentration inside the cell also rises over 1000-fold due to both a release of Ca2+ from the endoplasmic reticulum stores, and an influx of Ca2+ from the extracellular environment [12]. In addition, impaired mitochondrial function results in the increased generation of damaging oxygen free radicals that may exceed the cell antioxidant scavenging capacity [13]. Although a small amount of energy can be generated anaerobically extra-mitochondrially by the breakdown of glucose via glycolysis, the resultant formation of lactic acid lowers pH and triggers further cellular damage [11]. Unregulated ion movement, combined with slowed membrane pumps, results in interruption of the delicate intracellular ionic balance as well as osmotic cell swelling. Further reduction in temperature to below the freezing point can induce the formation of intracellular ice crystals that can physically damage cells further by puncturing membranes and disrupting intracellular structures. More importantly, growth of intracellular and extracellular ice crystals during freezing results in continued concentration of salt ions and shifting pH and salinity, that adversely, and sometimes irreversibly, impact intracellular and membrane proteins. Such cold-induced stresses, combined with a reduced ability to scavenge free radicals, can accumulate to levels that induce cell death. Furthermore, the non-frozen fraction during cryopreservation experiences a hypothermic continuum until reaching the vitrified state below the glass transition temperature. As such, hypothermic stresses are ever-present throughout the hypothermic continuum regardless of whether the sample is in the frozen or non-frozen state, and can contribute to the damage observed following cryopreservation [15]. Even cells that do not lyse during hypothermic/cryogenic temperature exposure are sometimes irreversibly damaged after a return to normothermic temperatures. A certain percentage of cells are damaged to the point that they will perish over time by necrosis, programmed apoptosis and secondary necrotic cell death in a process known as Delayed Onset Cell Death [16,17]. While cell loss can be mitigated by additional culture in the laboratory or cell manufacturing facility [19], expanded post-preservation culture would necessitate remote cell culture facilities at the clinic and a delay in patient administration, both of which add significant financial costs and could render the cell therapy non-viable from a commercial standpoint. In addition, cells that survive have likely undergone population selection, potential genetic drift and may exhibit adaptations that negatively influence downstream cellular function in clinical applications. Ultimately, after low temperature biopreservation, the ordered priorities of the cell are survival, repair and recovery, and then functional return. This is relevant in the context of cell therapy products, which are expected to function upon thaw and delivery to the patient. In practice, there are numerous stress points that occur during the workflow (Figure 1), and transition to and from low temperature biopreservation at both hypothermic and frozen conditions. For hypothermic biopreservation, critical steps include the choice of storage media at relevant stages, the rate and temperature of solution addition, the storage temperature, the warming rate and, ultimately, the removal or dilution of the storage solution. In addition, within a cell/ tissue manufacturing process, the variability of source material quality (apheresis/leukapheresis/tissue) and the collection/transition of cells from expansion/processing to final formulation/fill are increasingly recognized as potential bottleneck stress points in the overall workflow. Each of these stress points impacts post-preservation viability and functionality, and is deserving of detailed examination. However, for the purposes of this review, the focus on biopreservation media was chosen for further discussion as it constitutes an early and critical step in both hypothermic and cryogenic storage applications (Figure 2). The first consideration for an optimized biopreservation solution is to address the problems associated with the ionic imbalance.
. What is chronic gastritis ? | Health FAQs.
References
- Waldbillig DK, Quinn JV, Stiell IG, et al: Randomized double-blind controlled trial comparing room temperature and heated lidocaine for digital nerve block. Ann Emerg Med 26:677-681, 1995.
- Clark N, et al. High-energy ballistic and avulsive facial injuries: classifi cation, patterns, and an algorithm for primary reconstruction. Plast Reconstr Surg. 1996;98(4):583-601.
- Lichtenstein DA, Lascols N, Mezi Re G, Gepner AS. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med. 2004;30(2):276-281.
- Lyden PD, Shuaib A, Lees KR, et al. Safety and tolerability of NXY-059 for acute intracerebral hemorrhage: the CHANT Trial. Stroke 2007;38(8):2262-9.
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13.